What’s Going On With The COVID-19 Vaccine?
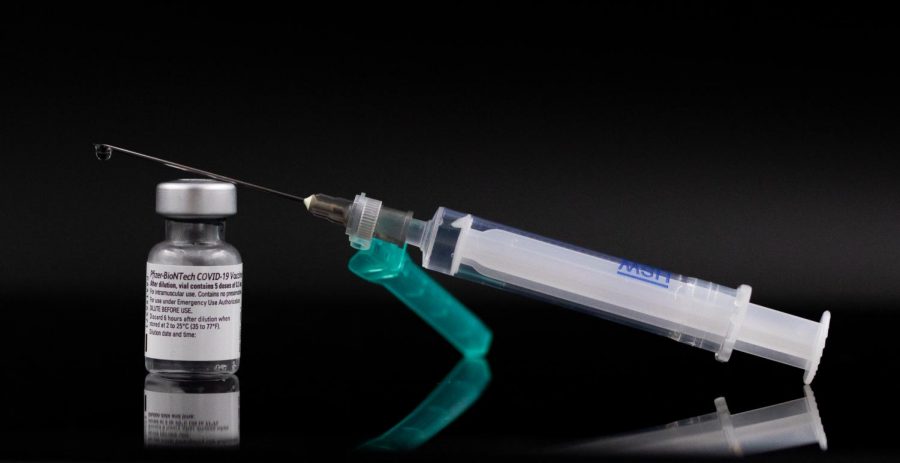
Courtesy of Arne Müseler via Wikimedia Commons
The Pfizer vaccine has been distributed to medical facilities.
The spread of the Covid-19 outbreak in mid-December marked a very important moment in this pandemic. However, the vaccine’s release sparked many questions in the media about Covid-19 and the vaccine itself. Here are a few common questions and their answers.
Covid-19 is a part of a family of viruses that can cause several illnesses such as the common cold, severe acute respiratory syndrome (SARS), and Middle East respiratory syndrome (MERS). In 2019, a new coronavirus was discovered, which came to be the cause of a disease outbreak in China. This new virus is called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This virus causes a disease called coronavirus disease 2019 (COVID-19).
Although sources cannot give an accurate time estimate of when the vaccines will be administered to everyone, the CDC says that the goal is to have the vaccine widely available when “large quantities are available for distribution.” The government is currently working with roughly 60% of U.S. pharmacies to deliver vaccines to the general population after the high priority groups have been vaccinated, according to The Wall Street Journal.
On December 20, 2020, the Advisory Committee on Immunization Practices released their provisional allocation recommendations for the distribution of the vaccine. In Phase 1a of the vaccination program, they recommended that the vaccine be distributed to health care personnel and residents of long term care facilities. In Phase 1b, it is recommended that the vaccine be given to people older than 74 and frontline essential workers (non-health care workers). Lastly, in Phase 1c, people in the ages between 65-74, people aged between 16-64 with high risk medical conditions, and essential workers not listed in Phase 1b will be administered the vaccine. Phase two advises people younger than 17 who are not already recommended in Phase 1a, 1b, or 1c.
Under the Trump Administration, the vaccine was free of charge for all Americans, and the same policy will continue under Biden’s presidency. The Centers for Medicare & Medicaid Services is “taking steps to ensure that all Americans will have access to the vaccine at no cost when it becomes available.” Even though the CDC clearly states that “vaccine doses purchased with U.S. taxpayer dollars will be given to the American people at no cost,” vaccination providers can charge an administration fee for giving someone the shot. Vaccination providers can be repaid for this by the patient’s private or public insurance company, or for patients who don’t have insurance, by the Health Resources and Services Administration’s Provider Relief Fund. However, it is illegal for someone to be denied the vaccine even if they can’t pay the vaccine administration fee.
The two vaccines currently available, Pfizer and Moderna, are “more than 90% effective at preventing symptomatic COVID-19” according to the CDC and the Infectious Diseases Society of America (IDSA). However, scientists are still uncertain whether they prevent people from spreading the virus to other people. Health-care professionals strongly suggest that people continue to wear a mask even after they have been administered the vaccine for preventative measures. Anne Rimoin PhD, MPH, professor of epidemiology at UCLA Fielding School of Public Health, tells Health Magazine that the vaccine trials only tracked how many vaccinated people become sick with COVID-19, which means that it is possible that “some vaccinated people could subsequently get infected but not develop any symptoms.” Rimoin further explains that those people could “transmit the virus without being aware of it, and the biggest risk is that they spread it to others who haven’t been vaccinated yet.” She ends her statement with a strong recommendation for everyone, vaccinated or not, to wear a mask.
Both of the vaccines have been under vigorous testing and studying to make certain that they are as safe as possible. The U.S. Food and Drug Administration has granted Emergency Use Authorizations for COVID-19 vaccines. If the vaccine wasn’t safe for human consumption, the FDA would not have approved it.